Selected publications Projects and grants
Team differentiation:
Migration of heavy metals in the environment
doc. Ing. Barbora Doušová, CSc.
Ing. Eva Bedrnová (PGS)
Mgr. Eliška Duchková (PGS) - kombinované
Ing. David Koloušek, CSc.
Layered double hydroxides and mixed oxides
prof. Ing. František Kovanda, CSc.
Ing. Michaela Dvořáková, Ph.D.
Szabolcs Muráth, Ph.D.
Migration of heavy metals in the environment
Due to their structure and sorption properties, some inorganic materials are used to remove toxic substances that are undesirable in the environment. Such materials are clay minerals, iron oxides and hydroxides or zeolites, but also biosorbents, eg biochar.
Especially arsenic, antimony and selenium, their geochemical properties, stability in waters and soils, transport through the environment.
 Symptoms of arsenicosis
Symptoms of arsenicosisTo decontaminate stressed areas, we use natural materials based on oxides of iron, aluminum and manganese, but especially aluminosilicates (clay minerals) in their original and modified form. Such materials are tested as selective sorbents suitable for removing toxic substances from soils and waters. We are also newly investigating biological sorbents (biochar), which promise perspective, cheap and ecological solutions in environmental protection.
 Original and modified clay sorbents
Original and modified clay sorbents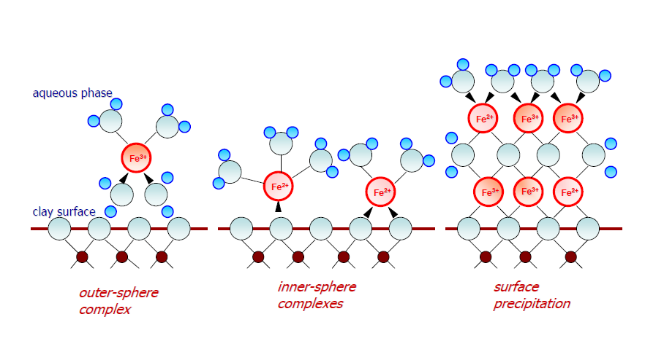
The second research area is focused on finding the optimal conditions for decontamination of a specific loaded system and designing the appropriate remediation technology.
 Leaks of acid mine waters in the Kutná Hora district (high concentrations of arsenic and iron)
Leaks of acid mine waters in the Kutná Hora district (high concentrations of arsenic and iron)
Concentrations of arsenic, antimony and selenium are measured by Atomic Fluorescence Spectrometry with Hydride Generation (HG-AFS) on a PSA 10.055 Millennium Excalibur instrument (manufactured by PSAnalytical, Kent, UK), which allows the determination of trace concentrations of these hydride-forming elements within the detection limit.

Semi-quantitative analysis of solid samples is performed by X-ray fluorescence spectroscopy (XRF) on a Rigaku NEX QC energy dispersive spectrometer (manufactured by Applied Rigaku Technologies, Inc., Austin, TX, USA) which allows the determination of elements from sodium (11Na) to uranium (92U) in solid substances, liquids, powders and alloys.

Zeolites
Zeolites are crystalline hydrated aluminosilicates of alkaline metals alkaline earth metals. The basic of zeolite structure is anionic frame of Si and Al T-atoms, which are tetrahedral coordinated with oxygen atoms. By reason of electrostatic forces it is not possible to make an Al-O-Al bond. Tetrahedrons form single or multipath circles, thereby cavities with diferent size originate in zeolite structure. These cavities are connected by channels. Non-frames cations are not fixed closely and can be changed for another cations. Zeolites are often used in ion-exchange reactions, have unique properties as sorbents and molecular sieves, and play important role in heterogenous catalysis. Zeolites offer in natural localities and a number of it was prepared synthetically.

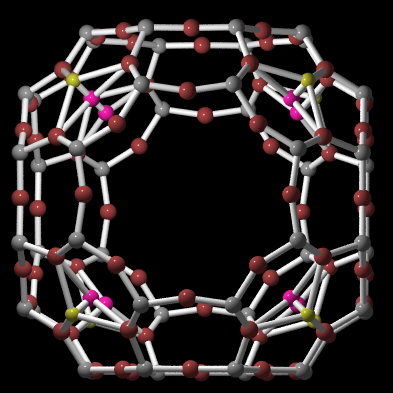
Structure of zeolites
In the Department of Solid State Chemistry, a synthesis technology was developed based on the hydrothermal reaction of fly-ashes (from power plants) and certified many use-possibilities of these zeolites (heavy metal cations separation, radioactive isotopes separation from wastewater, use in agriculture, etc.).
What do we study about zeolites?
- preparation of so-called geopolymer zeolites (zeolites A, X and P) from brick tablecloths
- separation of water from the water-ethanol system (preparation of absolute alcohol)
- influencing the sensory properties of wine by natural and synthetic zeolites
- recirculation systems for fish farming.
Layered double hydroxides (LDHs), known also as hydrotalcite-like compounds or anionic clays, represent a group of important inorganic materials usable in many applications. Their chemical composition can be expressed by the general formula MII1-xMIIIx(OH)2An-x/n·yH2O where MII and MIII are divalent and trivalent metal cations and An- is an n-valent anion. These compounds have a layered crystal structure composed of positively charged hydroxide layers [MII1-xMIIIx(OH)2]x+ and interlayers containing anions and water molecules. The value of x, usually in the range from 0.20 to 0.33, represents a portion of trivalent metal cations substituted in hydroxide layers. Layered double hydroxides exhibit anion-exchange properties; a weak bonding between the hydroxide sheets and interlayer anions enables their exchange for the other ones. At moderate temperatures (up to about 500 °C) layered double hydroxides are decomposed to form mixed oxides of MII and MIII metals. These mixed oxides are rehydrated in aqueous solutions; the rehydration process results in reconstruction of the layered LDH structure and intercalation of anions from the solution into interlayers. This unique property of layered double hydroxides can be employed for preparation of compounds intercalated with various anions and polar molecules or in removal of undesirable components from solutions. The delamination/restacking procedure, when LDHs are restacked from colloidal dispersion formed by their delamination in a suitable solvent, represents an interesting way for intercalation of water-insoluble components. The often used group name “hydrotalcite-like compounds” is related to the mineral hydrotalcite (Mg6Al2(OH)16CO3·4H2O). A group of other natural minerals with analogous crystal structure has been reported and a great number of synthetic compounds, combining various MII and MIII metal cations in hydroxide layers and various anions intercalated in the interlayers, can be prepared.
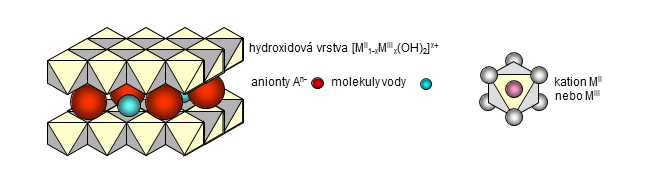
Structure of layered double hydroxides
Synthetic hydrotalcite is used mainly in the plastics industry, namely as a component of PVC stabilizing compositions and as a neutralizing agent (acid scavenger) in production of polyolefins. Layered double hydroxides can be applied also as nanofillers for synthesizing polymer-based nanocomposites, in which inorganic nanoparticles dispersed in relatively low concentration in the polymer matrix improve its properties. The representative pharmaceutical application of layered double hydroxides is the hydrotalcite-derived antacid. They are also studied as carriers for drugs and other bioactive substances. Layered double hydroxides are widely used in heterogeneous catalysis, mainly as precursors for preparation of mixed oxide-based catalysts. The anion-exchange properties of layered double hydroxides and their ability to recover the layered crystal structure during rehydration of thermally decomposed products may be utilized for adsorption of undesirable contaminants. Layered double hydroxides are often used also as host inorganic structure suitable for intercalation of various anions and molecules, resulting in the preparation of hybrid materials with interesting physical and chemical properties.
Our interests
Preparation of precursors and mixed oxides for heterogeneous catalysis
Our research is focused on preparation of layered double hydroxides and other precursors of desired chemical composition and thermal treatment of these precursors including a study of the thermal decomposition, formation of oxide phases and their transformation during heating. We are interested also in the deposition of precursors and mixed oxides on metal and ceramic supports. The obtained materials are then studied as catalysts for removal of gaseous pollutants, namely the volatile organic compounds and.
Preparation of layered double hydroxides intercalated with organic components
We are concerned with synthesis of the host structures and their intercalation with organic anions or molecules, especially the active pharmaceutical ingredients; this research is focused on development of new solid dosage forms. The layered double hydroxides intercalated with organic components are studied also in other applications such as preparation of LDH/polymer nanocomposites and photoactive materials. We are interested also in synthesis of other organic-inorganic hybrid materials.
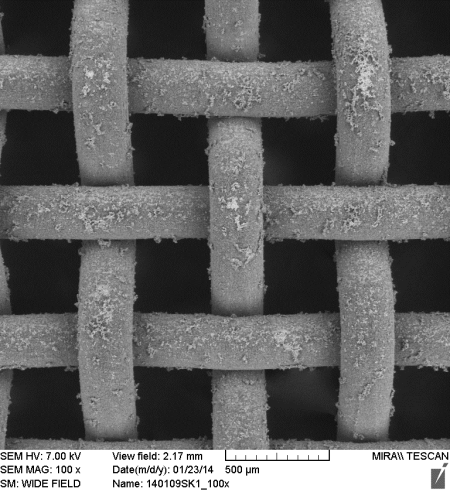

Supported catalyst with active layer of Co-Mn-Al mixed oxide obtained by thermal decomposition of LDH precursor prepared on anodized aluminum mesh
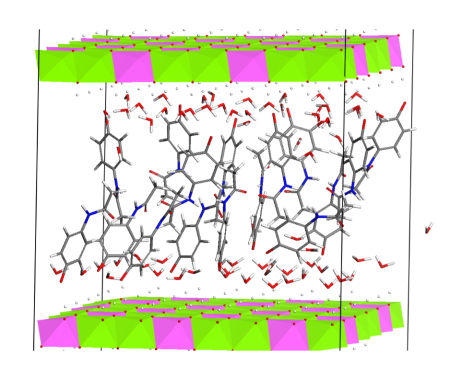
Arrangement of paracetamol molecules intercalated in the LDH interlayer
